26.01.2022 – Total alkalinity is a measurement of the concentration of all alkaline substances dissolved in the water that can both attract and release hydrogen ions. Total Alkalinity is analyzed by precise, open-cell, potentiometric titration using a semi-automatic total alkalinity analyzer developed and provided by Andrew Dickson and his team from the University of California, San Diego. Persons responsible for these analyzes are Aleksandra Winogradow, Ph.D, and Magdalena Diak, Ph.D.
 |
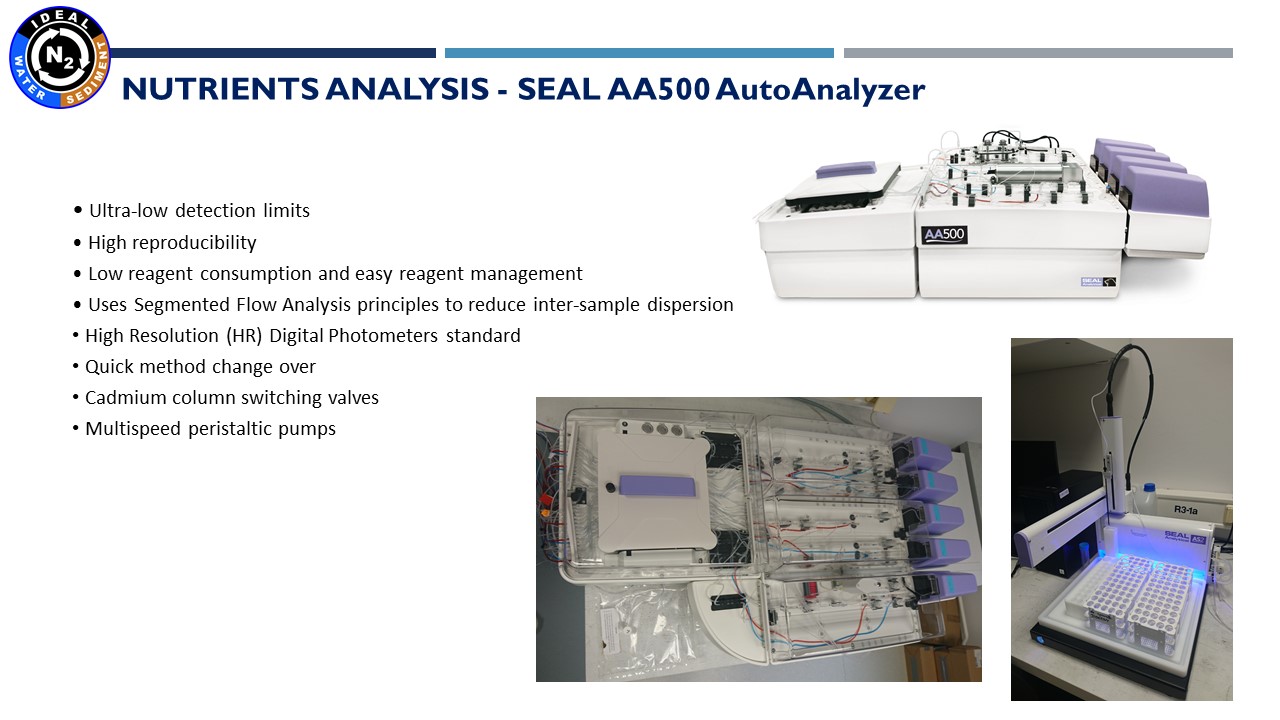
12.01.2022 – Analyzes of nutrient concentrations are performed using an automatic SEAL AA 500 analyzer. This device uses basic colorimetric methods – the intensities of the colors of the obtained complexes are then measured in a spectrophotometer. Our analyzer has 5 channels that enable for simultaneous measurements of PO42-, SiO2, NO2-, NO3-, NH4+. Quantitative analysis is based on the use of a calibration curve. Person responsible for these analyzes is Marta Borecka, Ph.D.
 |
15.12.2021 – The DOC analysis are performed in TOC-L analyzer (Shimadzu). In order to remove inorganic carbon a filtered and acidified water sample, before measurement, is sparged with synthetic air. The method is based on oxidation method with Pt catalyst in high temperature (680°C). Non-purgeable organic carbon compounds are combusted and converted to CO2, which is detected by a nondispersive infrared detector (NDIR). Person responsible for these analysis is Aleksandra Winogradow, Ph.D.
 |
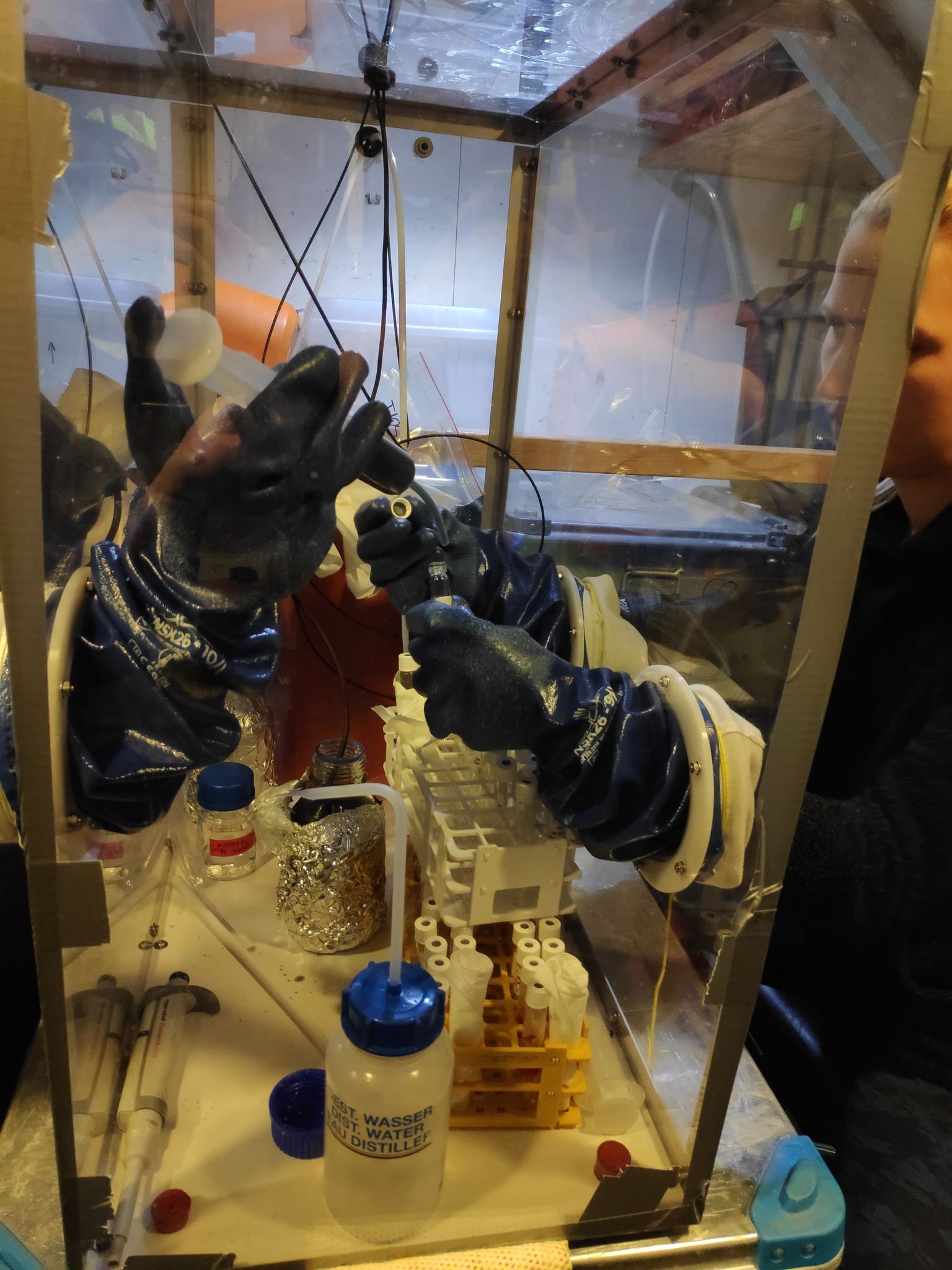
01.12.2021 – Gas Bench II is the combination of GasBench interface and Isotope Ratio Mass Spectrometry. While with the use of MIMS we can measure mainly 14N, with Gas Bench we can measure all of the nitrogen isotopes. Hence, in the incubation experiments, which we are performing, we can add different 15N standards and use them as a tracers. What it can give us? We can distinguish which process is observed in our samples – is it denitrification or anammox, or other reactions as nitrification and DNRA. As you can see, samples are prepared in the Glove Box filled with helium, which protect them from the contamination with nitrogen from atmosphere. Person responsible for these analysis is Magda Diak, Ph.D.
 |
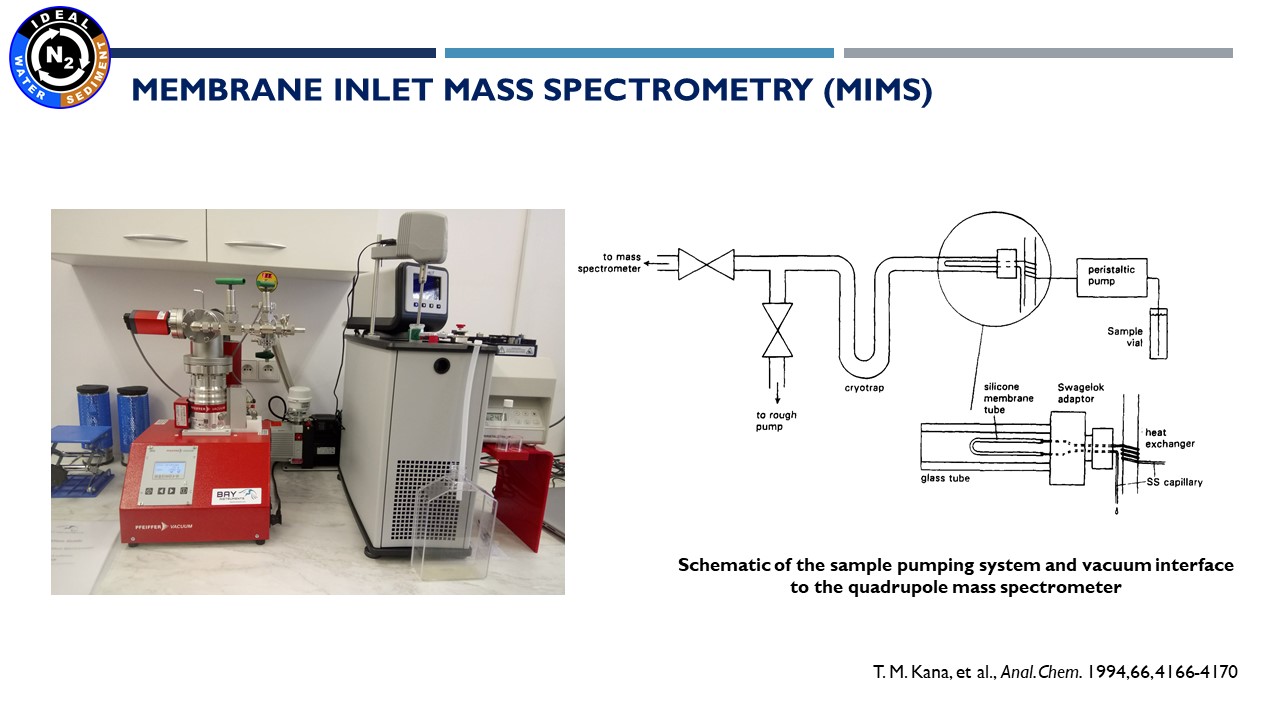
18.11.2021 – the results of the denitrification and anammox reactions (the main processes which we are focused on in our project) is the production of gaseous nitrogen. To measure its concentration we are using a technique named - Membrane Inlet Mass Spectrometry. This technique allows to perform rapid and high-precision measurements of dissolved nitrogen, oxygen and argon in water. Person responsible for these analysis is Marta Borecka, Ph.D.
 |
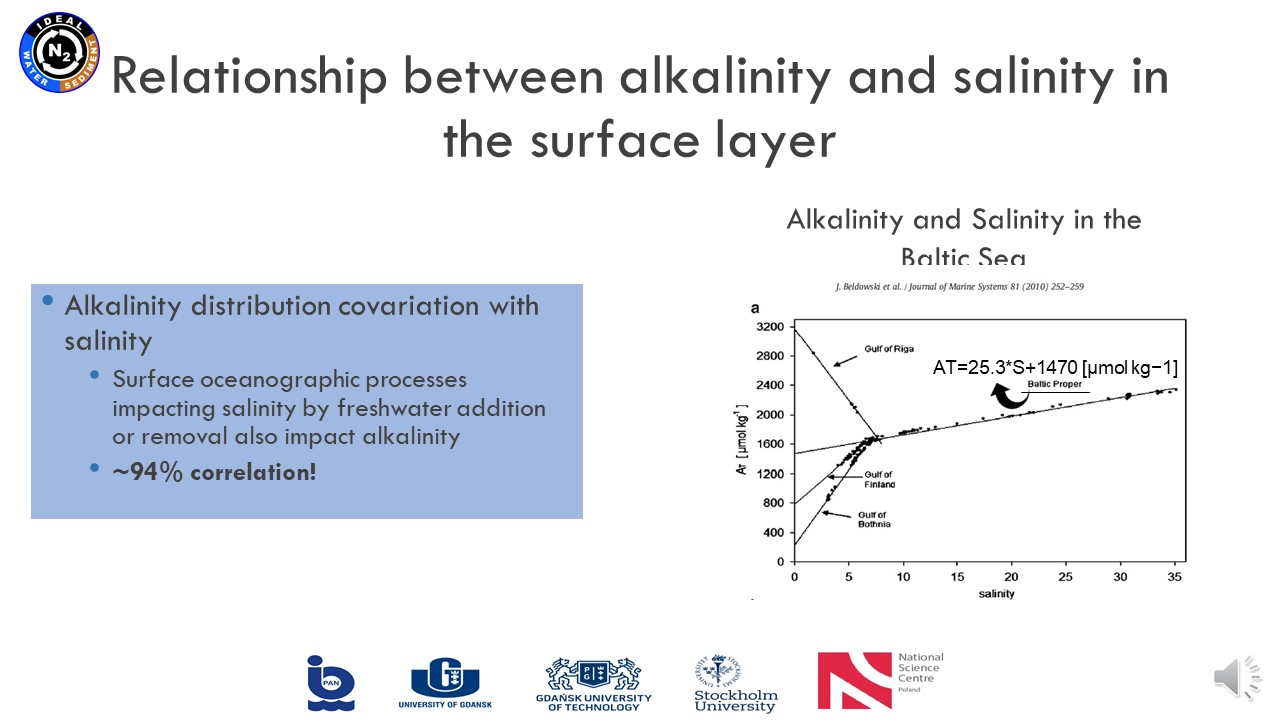
June 17, 2021, Karina Vieira Konoplianyk MSc - presentation: "Alkalinity Distribution in the Baltic Sea"
 |
 |
 |
 |
 |
 |
 |
 |
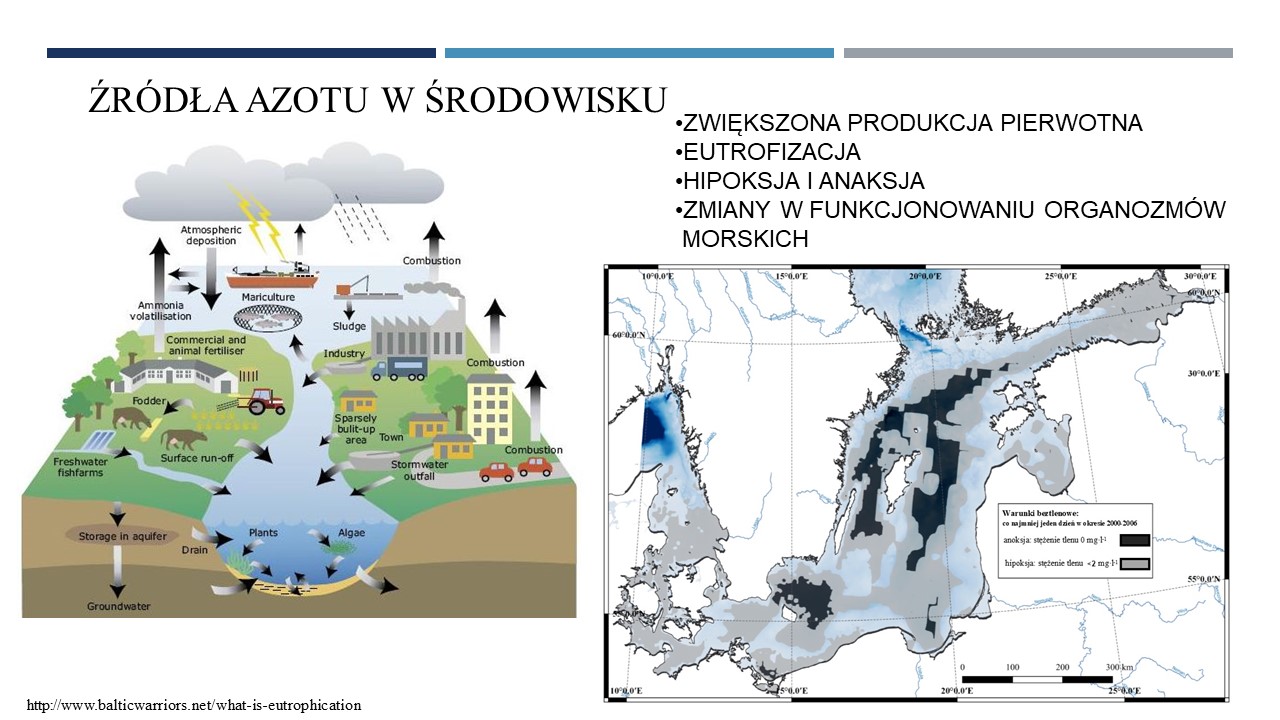
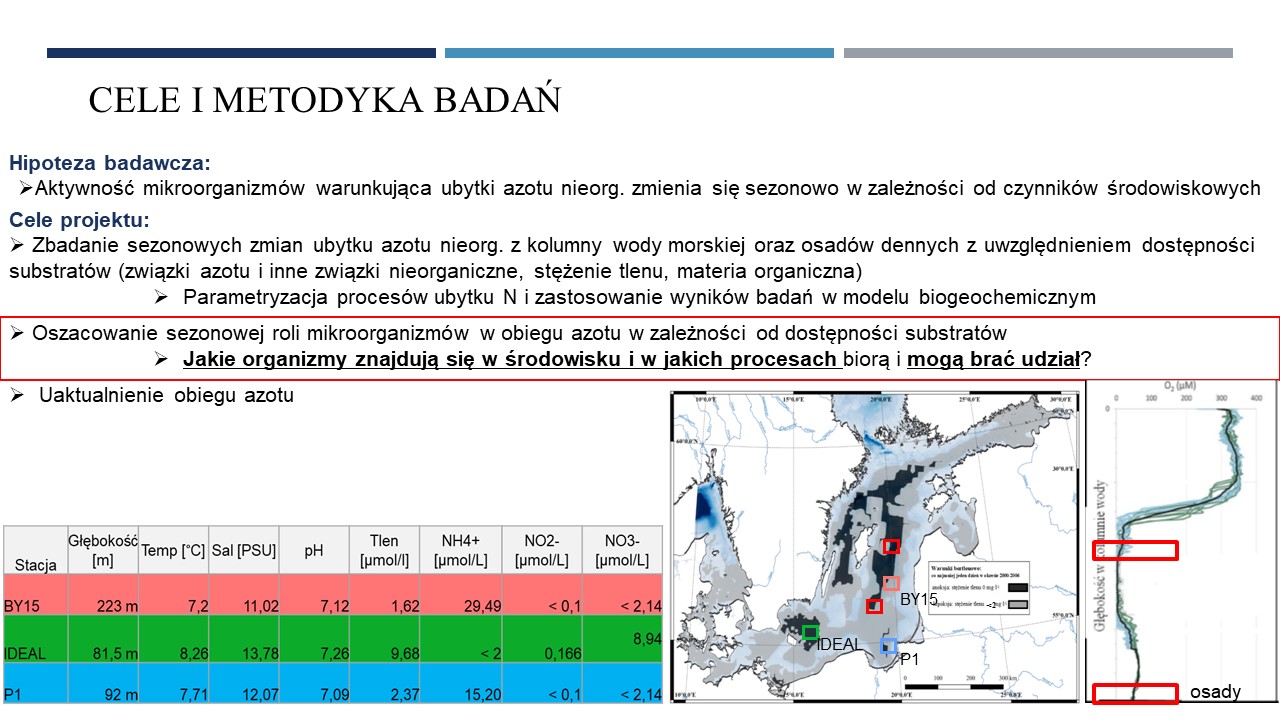
11.06.2021 - Michał Grabski MSc: "Genomiczna analiza mikrobiologicznych procesów obiegu azotu w trzech głębiach Morza Bałtyckiego"
 |
 |
 |
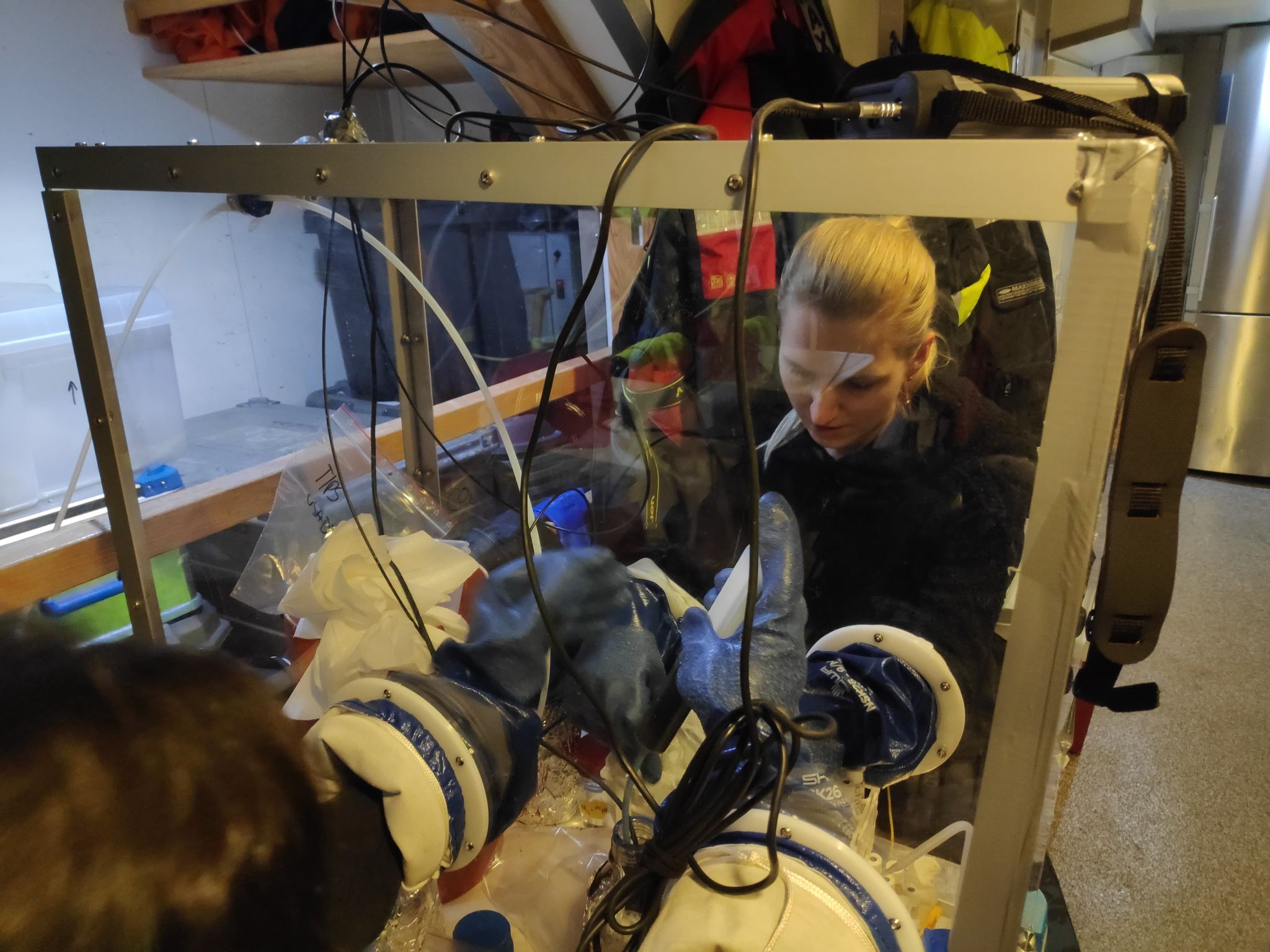
Another cruise in the IDEAL project – 10-25 April 2021. We present our new system for samples collection and preparation - GloveBox.
 |
 |
 |
 |
 |
 |
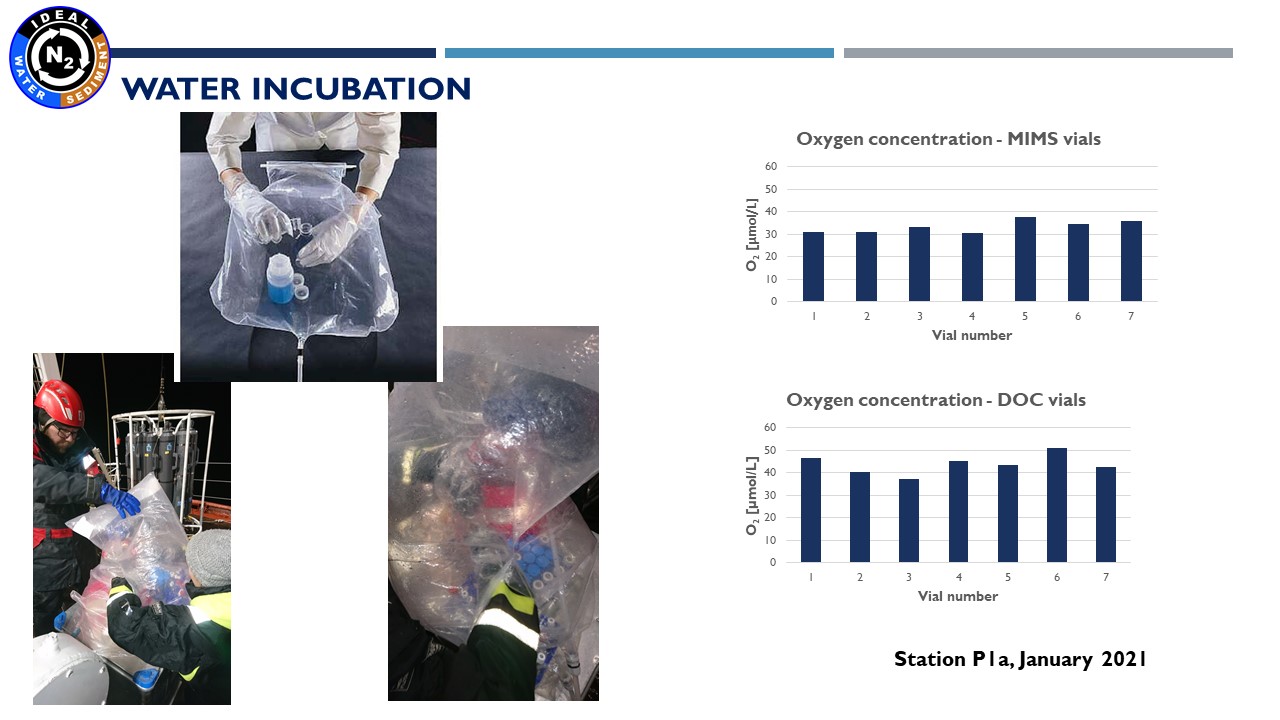
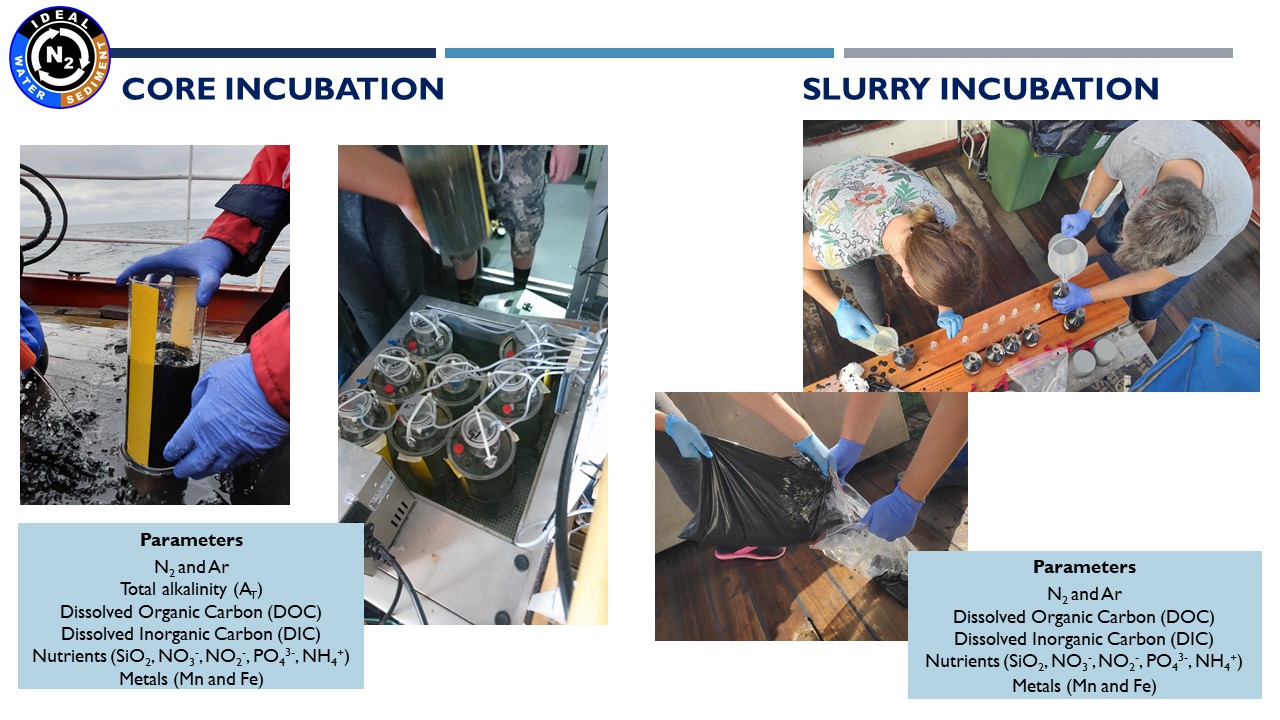
Presentation: "Methodology of water and sediment incubation for the estimation of denitrification. Preliminary results in IDEAL project"
(24.03.2021, Marta Borecka Ph.D.)
 |
 |
 |
 |
 |
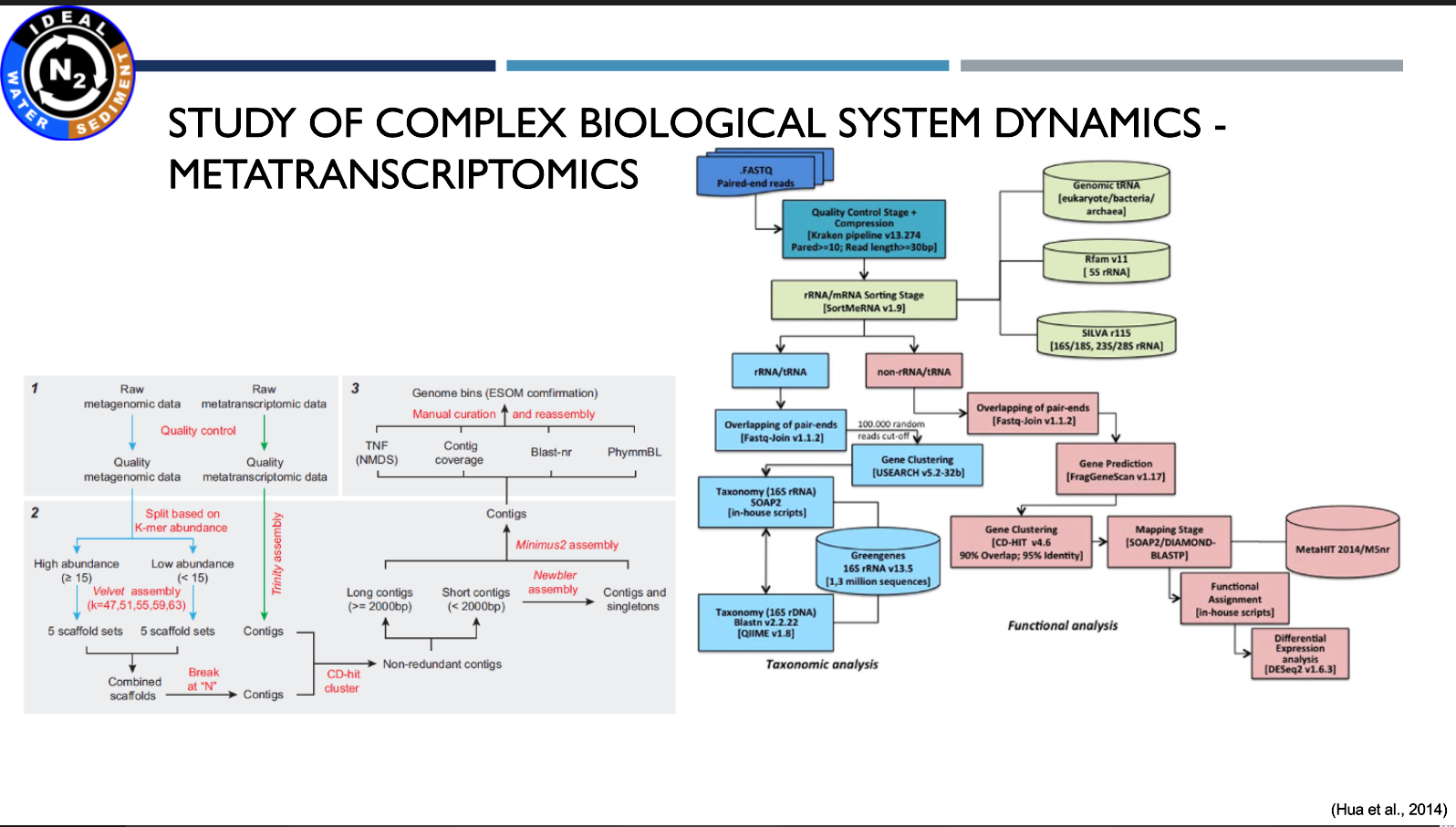
Presentation: "The utilisation of metagenomic and metatranscriptomic tools to solve environmental relations"
(17.03.2021, Michał Grabski M.Sc.)
 |
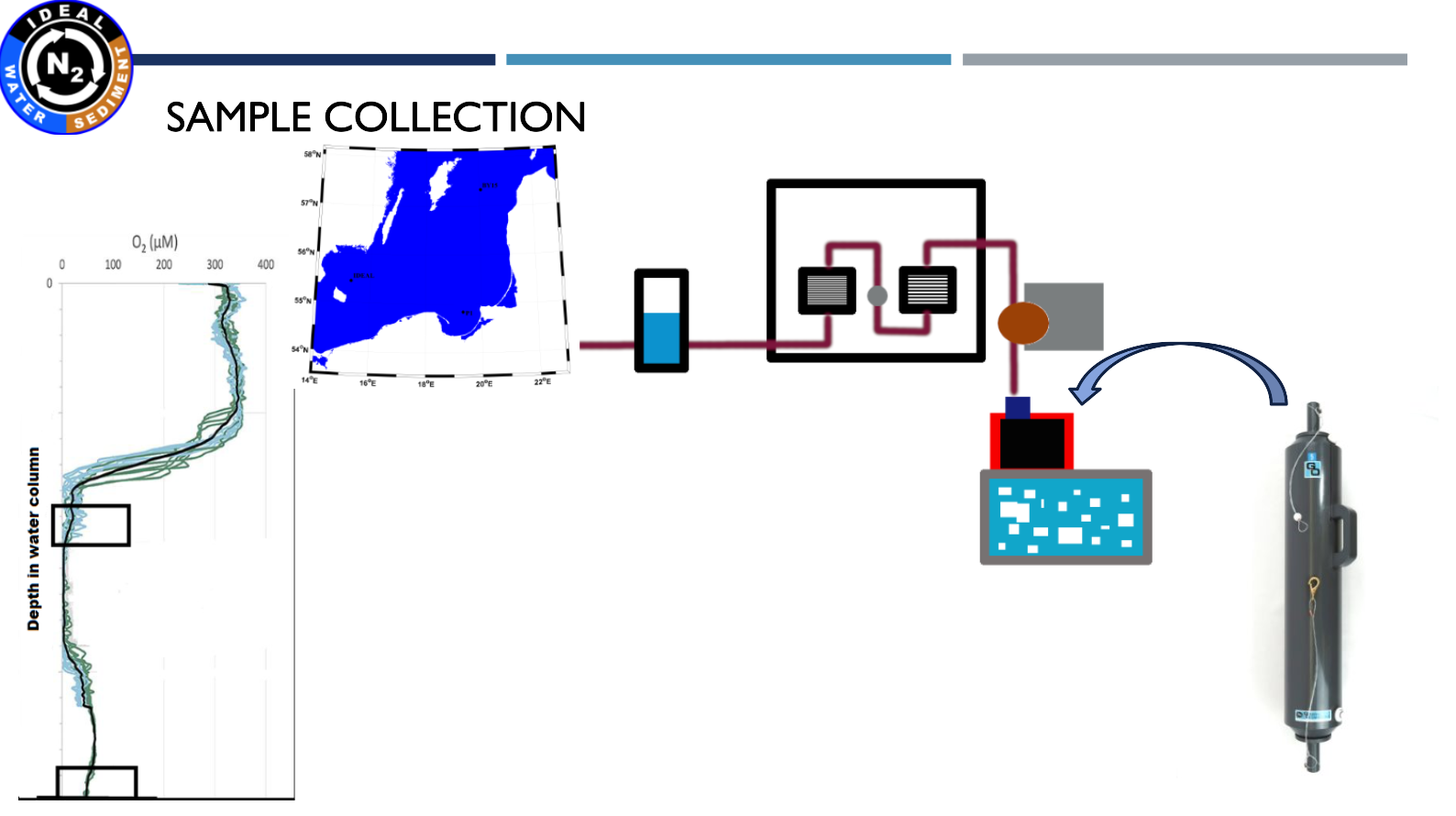 |
Presentation: "IDEAL project - characterization and preliminary results" (10.03.2021, Beata Szymczycha Ph.D.)
 |
 |
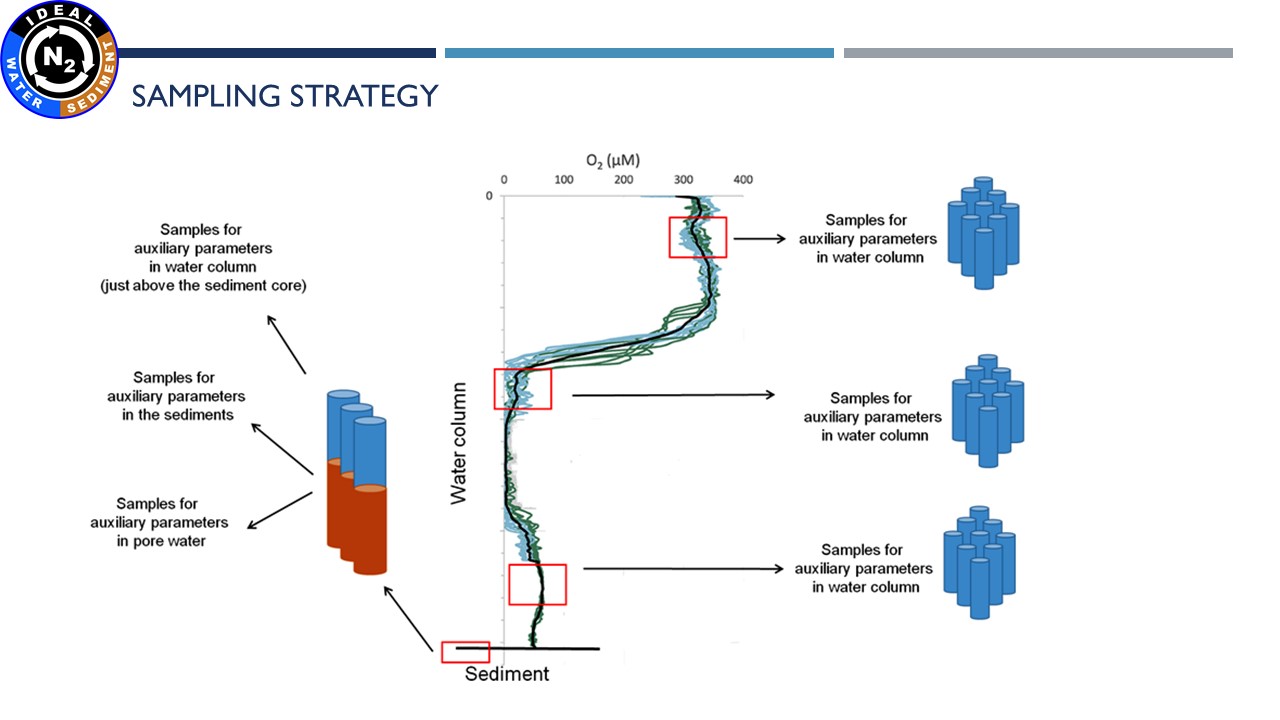 |
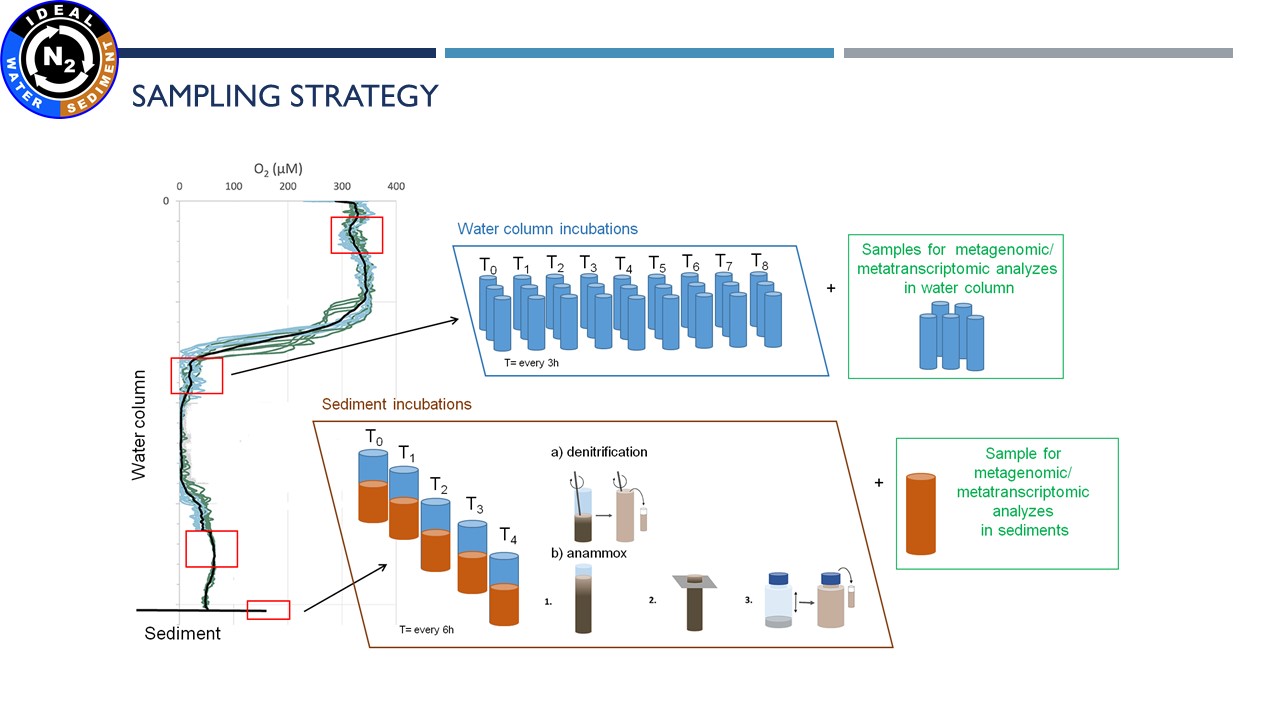 |
Presentation: "From polish mires to the Baltic Sea – a nitrogen story" (03.03.2021, Karina Vieira Konoplianyk M.Sc.)
 |
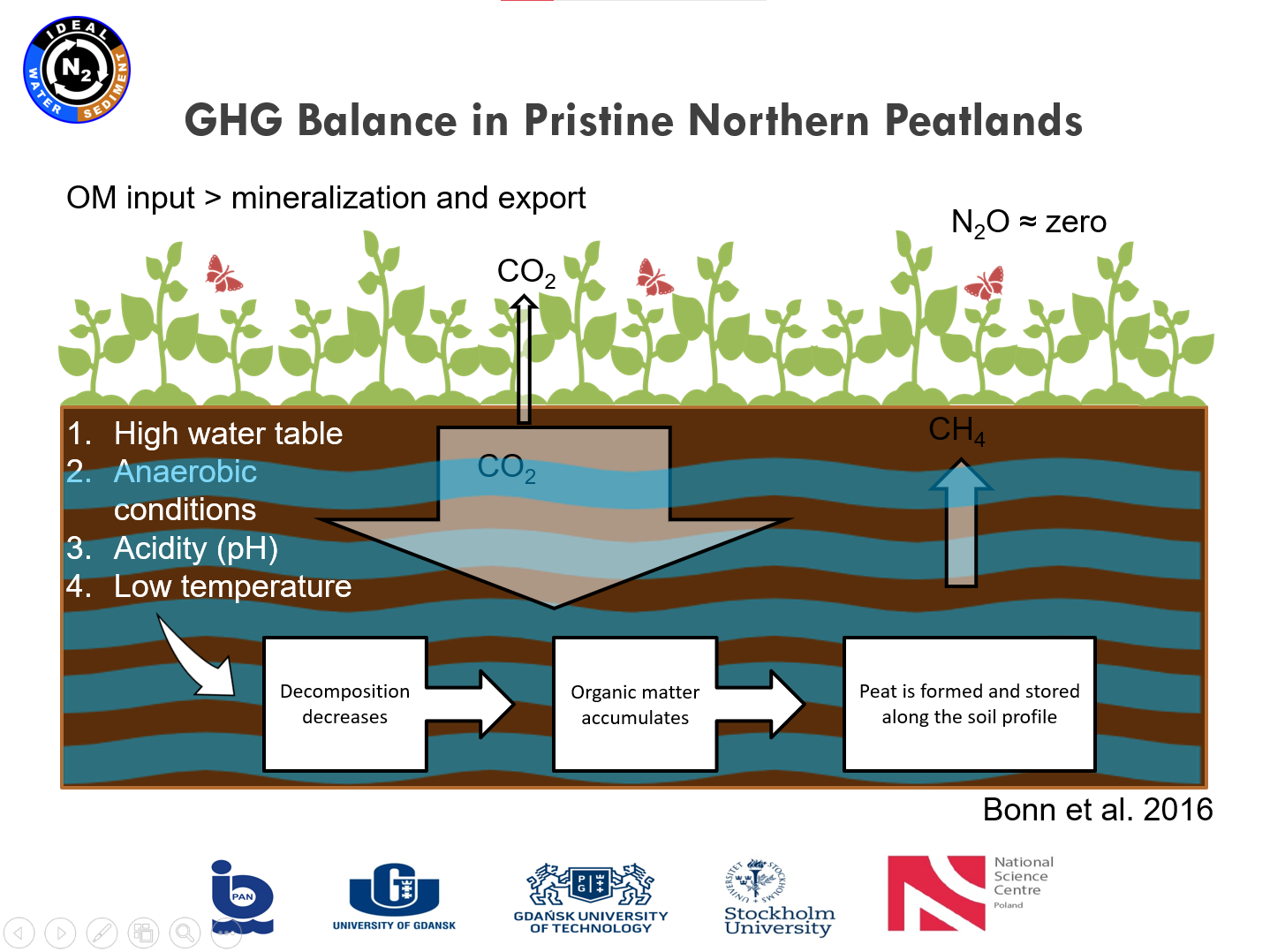 |
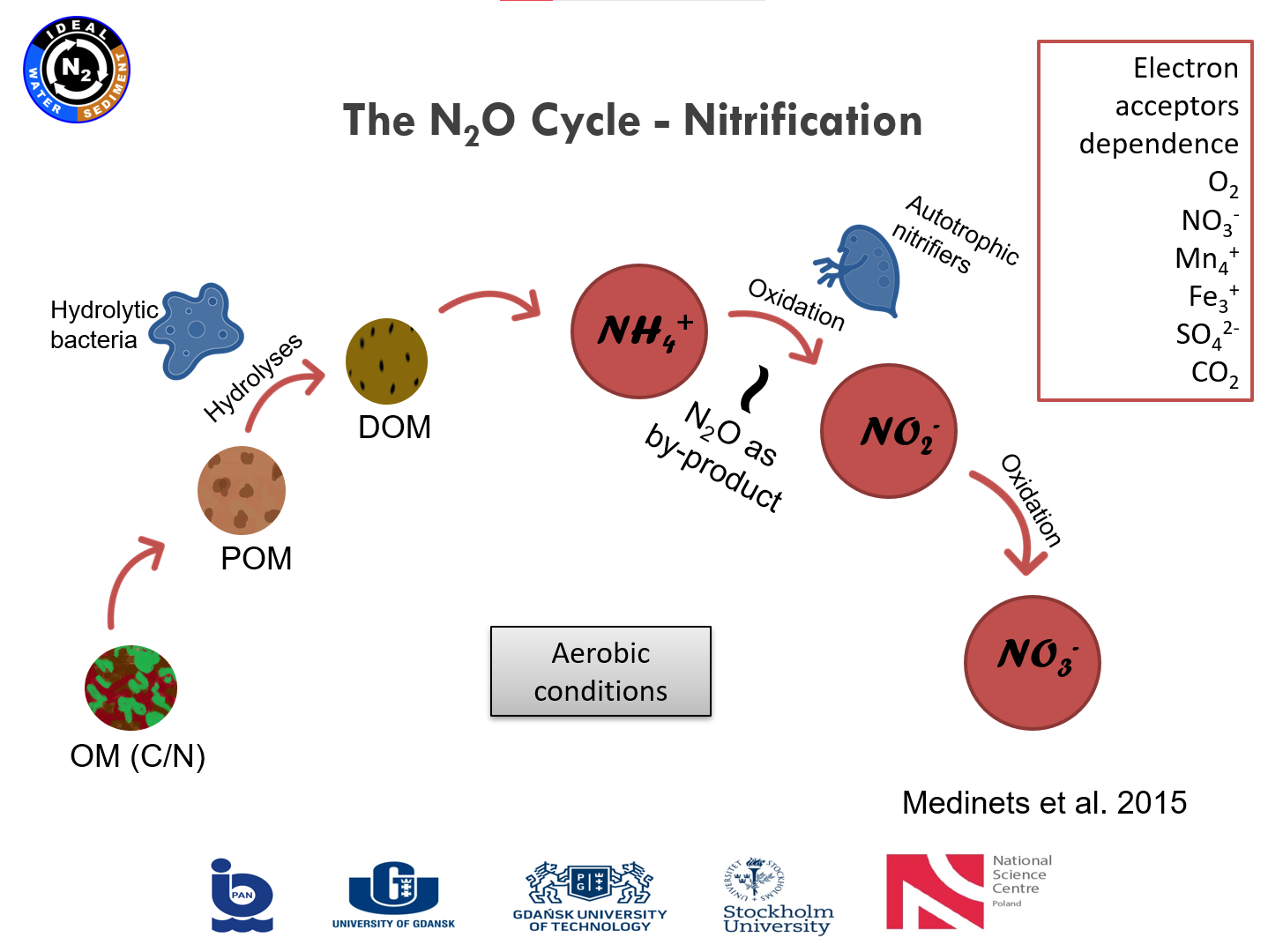 |
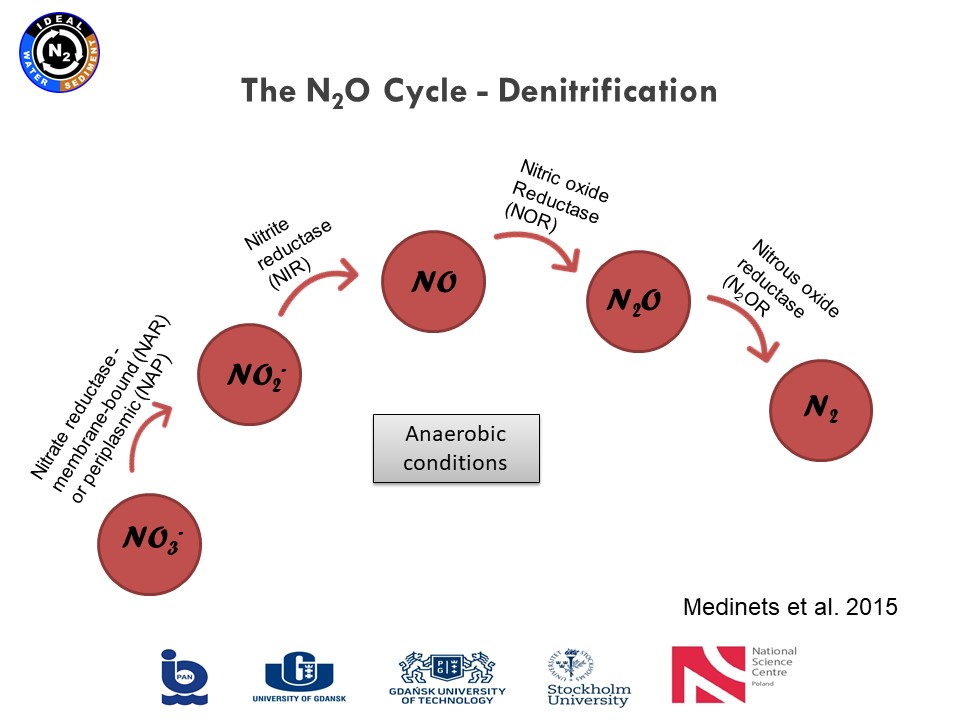 |
 |
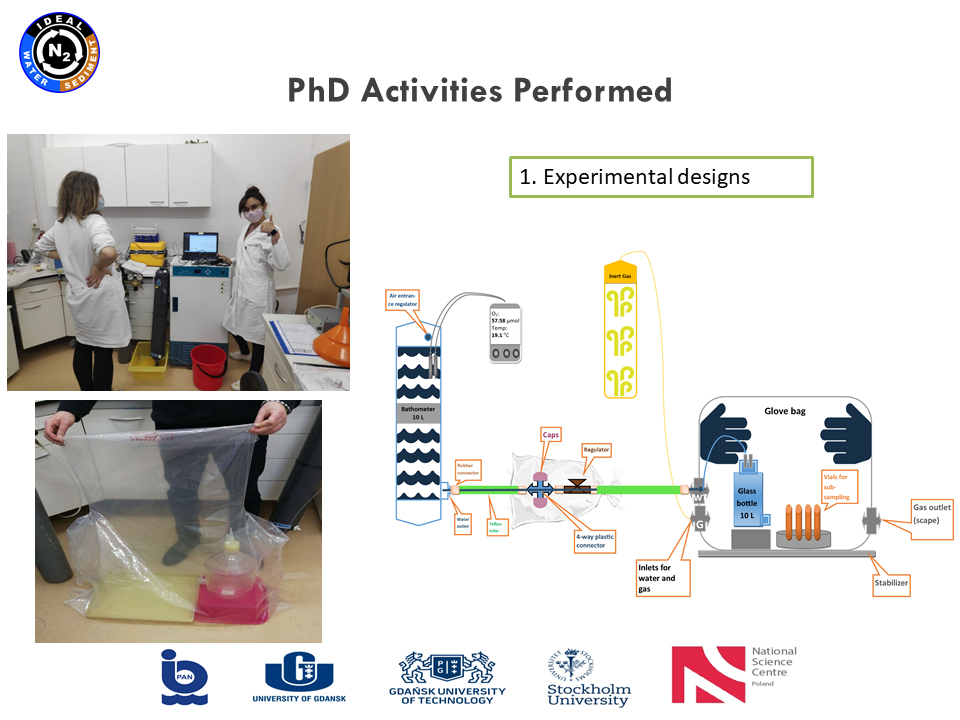 |
Second sampling campaign in the IDEAL project - 08-21.01.2021. Our system - Glove Bag filled with helium - works! We managed to collect samples without oxygen contamination. However, as you can see, it is not easy to work in it.
 |
 |
 |
 |
 |
 |
 |
 |
December 2020. During our first research cruise we encountered a problem related to the contamination of samples with oxygen. The incubation experiments performed during cruise should mimic the environmental conditions. We sample anoxic water, as denitrifying microbes require a very low oxygen concentration. Therefore we should take a special care while sampling, in order not to increase the oxygen level. For this purpose we had to create a system filled with inert gas. It works in the laboratory, so we hope it will also work in the field.
 |
 |
 |
 |
November 2020. Preparations for our next cruise are going on. We test the sample filtration system for metagenomic and metatrancriptomic analyzes. This will allow us to study what bacteria live in the samples we collect and what their tasks are.
October 2020. Our PhD student Michał has just started DNA isolation tests from the material collected during the September cruise. This is the next stage of metagenomic analyzes that will allow us to identify and characterize microorganisms present in the collected samples. Thanks to this, we will be able to assess the effect of bacterial activity on the nitrogen removal processes in the sediment and the seawater column.
| The sampling stations were located on Bornholm Deep, Gotland Deep and Gdańsk Deep. |







|
| Cores preparation and incubation |

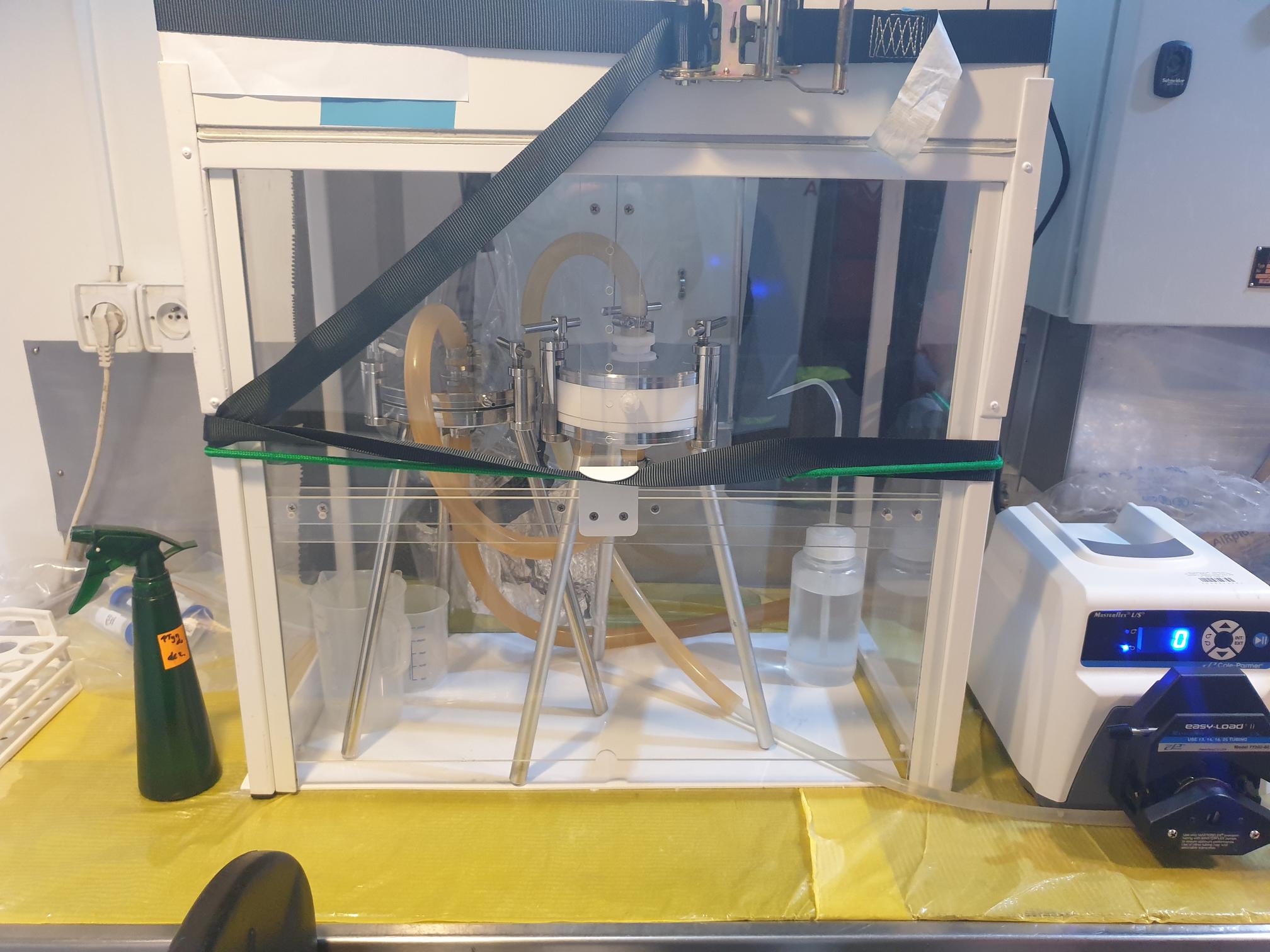

|
| Sampling and filtration of water for metagenomic and metatranscriptomics analysis |
  |
  |
 |
 |
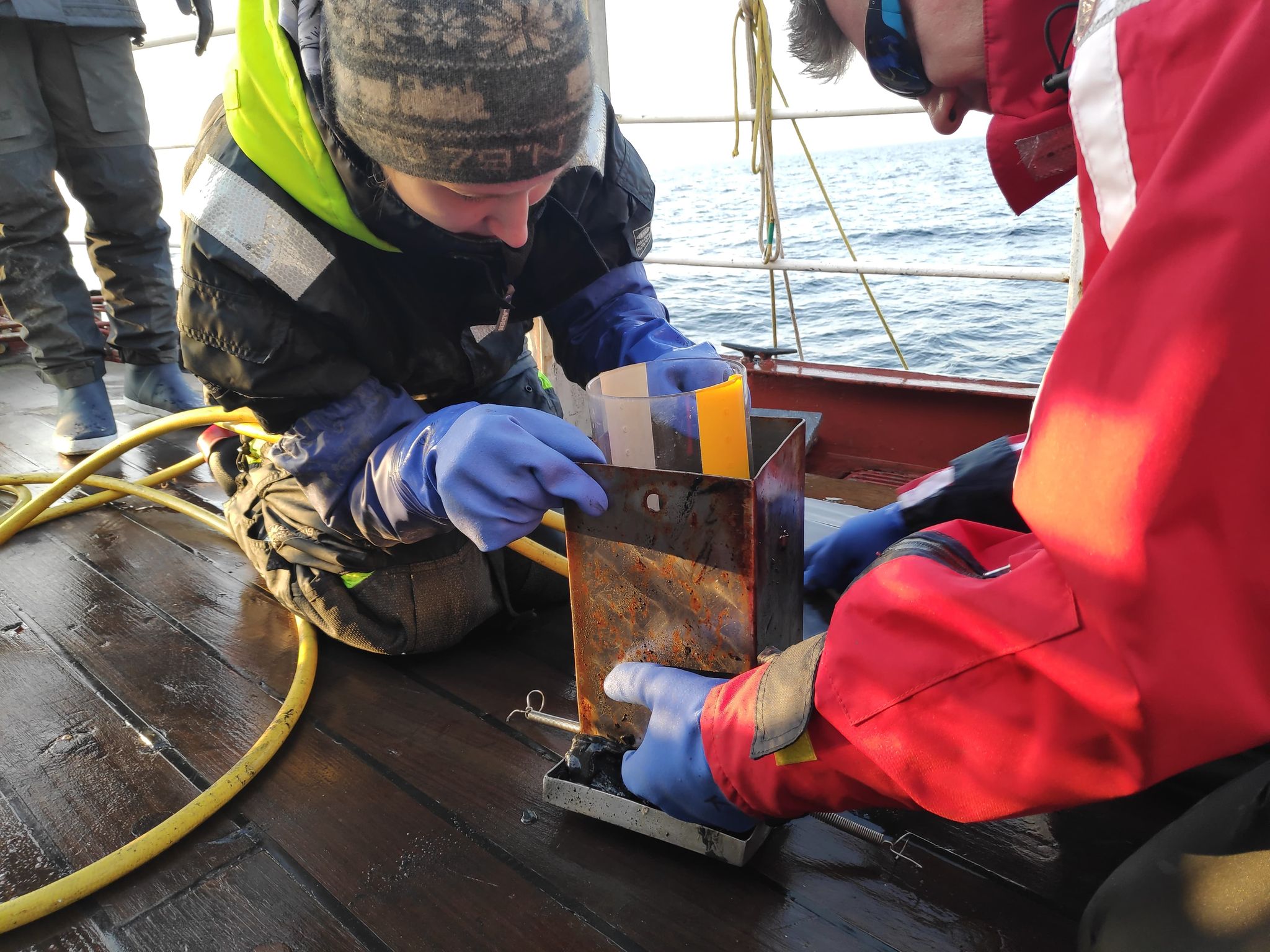
| Sediments preparation for incubation | Pore water sampling | Sediment core slicing | Incubation of water samples |
 Membrane Inlet Mass Spectrometer (MIMS) |
 Total Organic Carbon Analyzer TOC-L (Shimadzu) |
 Inductively Coupled Plasma Mass Spectrometer (ICPMS) ELAN Sciex 9000 (Perkin Elmer) |

 Isotope Ratio Mass Spectrometer combined with Flash EA 1112 Series (Thermo Scientific) |