|
Abstract
Objectives Methods Working plan Work packages References |
MethodsThe methods will involve field sampling, experimental studies, body-, cell- and genome size analysis, deep sequencing of genomes for selected taxa as well as the size spectra analyses of whole communities applied for selected limnetic, terrestrial and marine species and communities along a thermal gradient from temperate to high Arctic areas.The main approach will be to first determine the adult body size under various temperature regimes, and secondly to analyse cell size and genome size. For vertebrates (fish) this will be done by blood samples analyses on flow cytometer. Since fish have retained nuclei in their erythrocytes, this will provide information on genome and cell size in the same samples, and erythrocyte volume in generally assumed to reflect overall somatic cell size, although we will test this assumption by also screening muscle tissue in selected samples. In insects, the size of individual eye (ocellus) has been shown to be a good proxy for cell size. We will explore if this may be used also for crustaceans. For analysis of genome size in invertebrates whole animals will be resolved in buffer and run on flow cytometer. Both groups and methods are currently run at UiO. The deep sequencing will be run for 2-3 selected species where strong thermal responses in genome size is identified. The sequencing will be run with 454 and Illumina at the CEES sequencing platform, and bioinformatics will focus on eventual proliferation of intron regions in expanded genomes (repetitive elements, transposons and retrotransposons). The response of organism body size to the changing thermal regimes will be explored both at the scale of the selected species populations and of the whole communities. Populations and communities will be sampled at sites located along the gradients of changing thermal conditions. For marine, limnetic and terrestrial systems these will include different sites on Svalbard and mainland Norway, representing a latitudinal and thermal gradient (for details, see the respective WPs). Sampling in lakes will be done by gillnets and net-hauls. Marine organisms will be collected from board of research vessels r/v Oceania and r/v Helmer Hanssen. Mesozooplankton will be collected in stratified-vertical net hauls (MPS, 180 µm net mesh) from the bottom to the surface. Soft bottom benthic fauna (macro- and meiofauna) will be collected with use of van Veen grab and box-corer. Calcareous colonial fauna will be collected with use of scuba diving techniques. 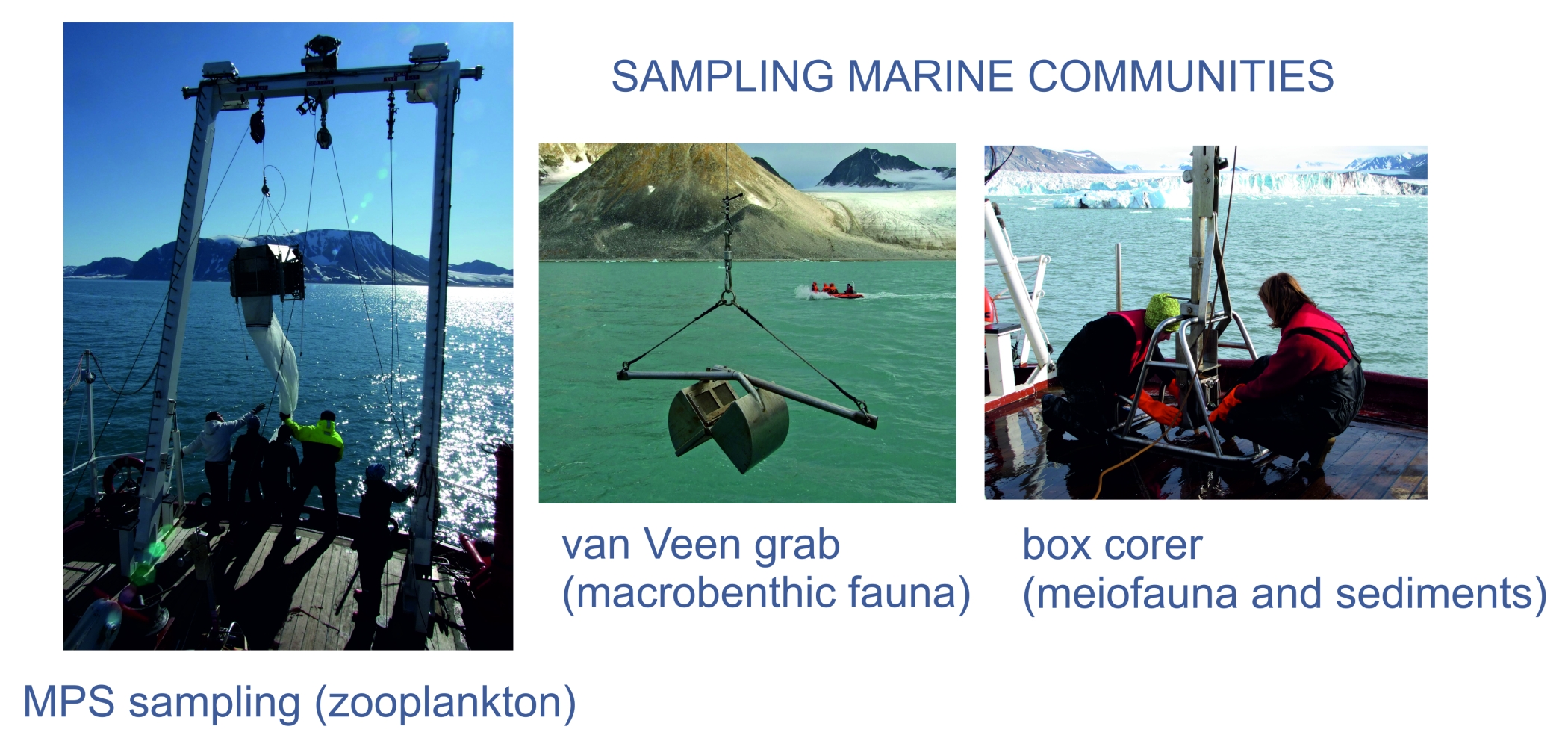
In addition to the analyses based on present day populations, the temporal changes in body size will be studied for Bryozoa (based on historical collections spanning XX century) and Foraminifera (based on Holocene sediments). Paleontological record of Foraminifera test size changes will be analysed in three cores collected with use of gravity corer in Svalbard waters. 
Normalized Biomass Size Spectra approach will be applied to assess the size distributions in pelagial and benthic communities in the sea. To determine NBSS all animals in a sample will be identified and measured under a dissecting microscope using computerized image analysis system. Organism dimensions will be measured and used to calculate biovolume from published relationships. Individual wet weights will be determined from the animal volume and converted into a Ash Free Dry Weight (AFDM) according to published formulae. Biomass Size Spectra will be constructed using log groupings of invertebrate dry/size weight (μg)/ on the X-axis and total biomass per class on the Y-axis. The slope of the observed biomass spectra will be calculated by standard linear fitting (least-squares).The comparisons of NBSS among samples and sampling sites and analysis of their relationships to environmental data will be performed with use of statistics. 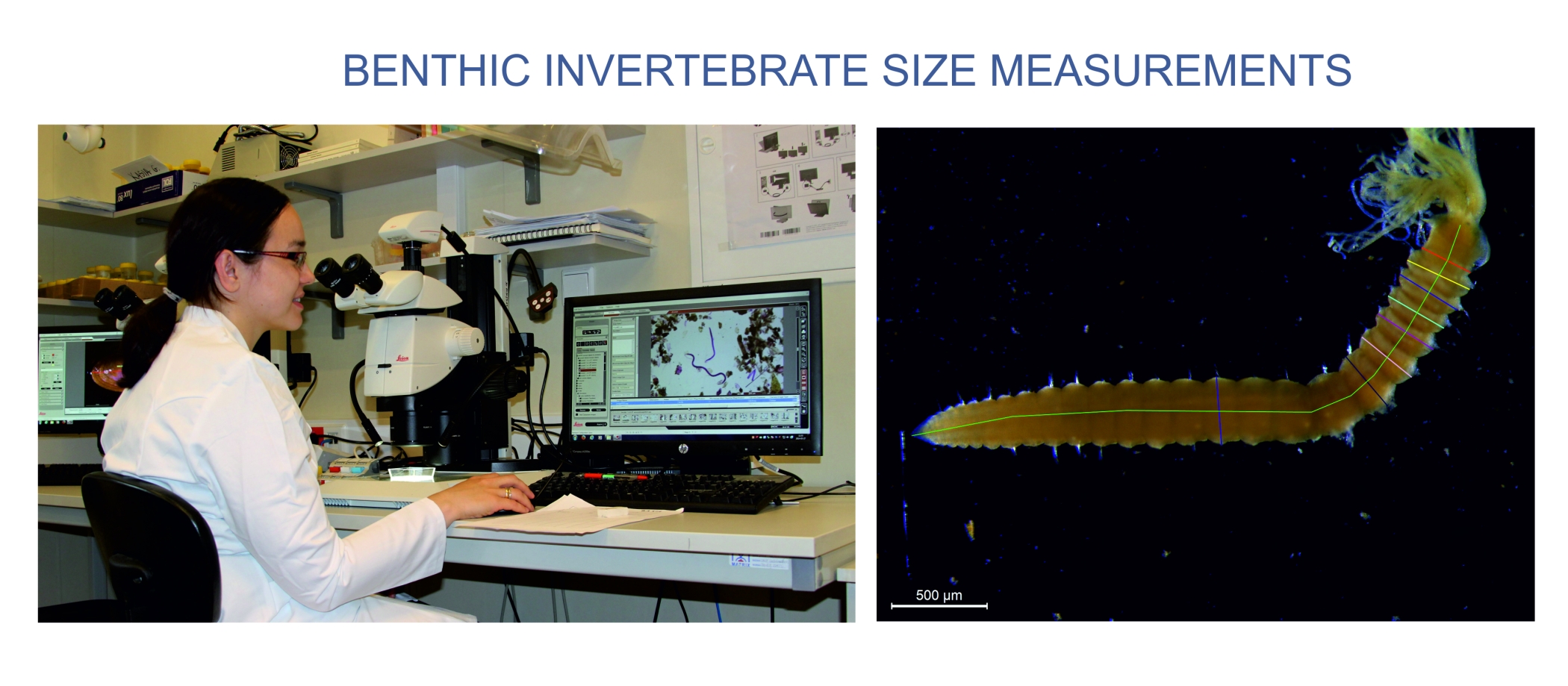
NBSS in benthic communities will be based on data derived from measurements of organisms in collected samples, while for pelagic communities will be based mostly on a high-resolution measurements by the Laser Optical Plankton Counter (LOPC). LOPC was designed for automatic counting and sizing plankton particles for fine- and mesoscale zooplankton studies. It provides continuous data on zooplankton abundance, size structure and distribution which are collected simultaneously with environmental parameters (temperature, salinity, chlorophyll fluorescence). In our project application of LOPC will be accompanied with net sampling and taxonomic analyses, which will provide the data on species representing particular size-modes in size spectra. 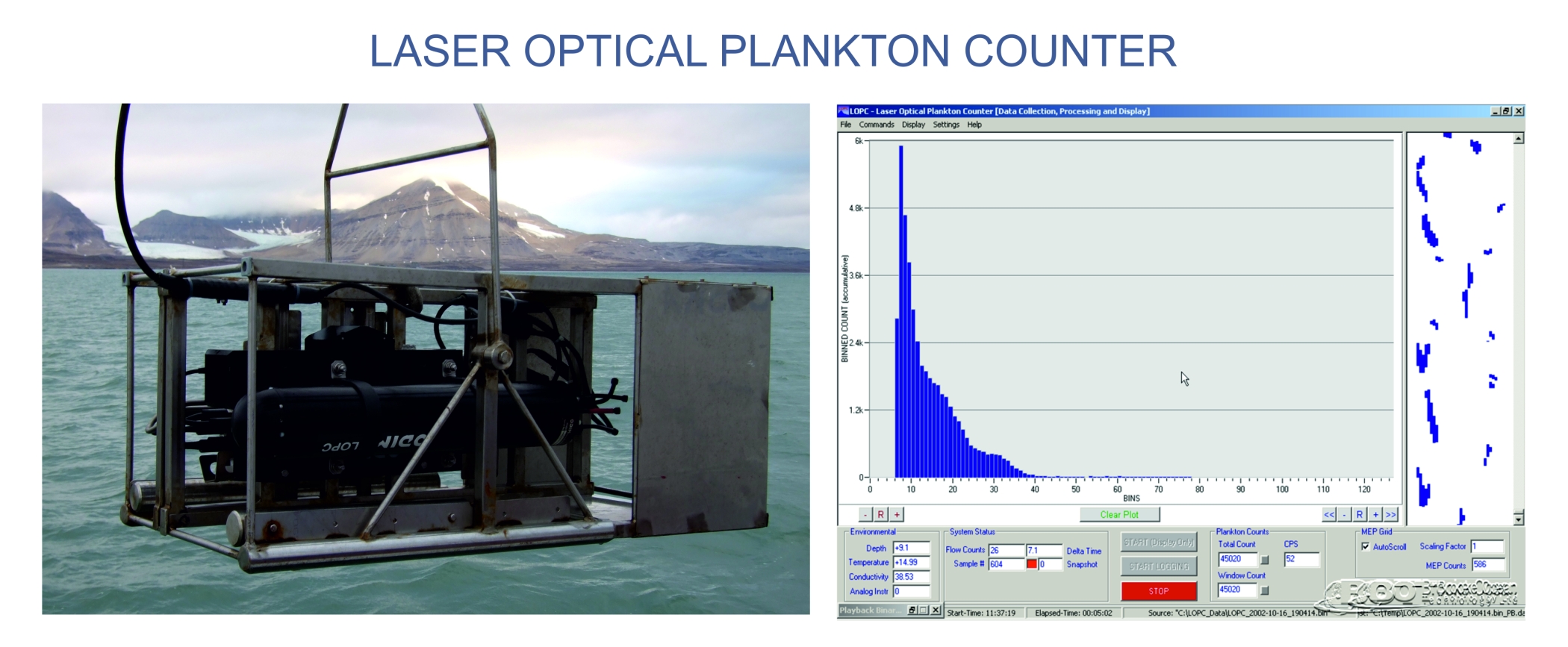
For both benthic and pelagic communities the obtained NBSS will be applied to assess secondary production (using published formulae based on the allometric relationship between weight and P/B). |





